Abstract
Introduction: Hypomethylating agents (HMAs) are recommended as standard of care treatment for patients with higher-risk myelodysplastic syndromes (MDS); however, intravenous (IV) and subcutaneous (SC) administration of HMA therapy has been associated with additional patient burden. In July 2020, the HMA oral decitabine and cedazuridine (DEC-C) was approved by the US Food and Drug Administration (FDA) for the treatment of MDS, as an alternative to IV or SC HMAs. This study reports initial results evaluating DEC-C and IV/SC HMA treatment patterns and population characteristics among MDS patients in a real-world setting.
Methods: This retrospective observational study utilized the IQVIA PharMetrics® Plus database which comprises adjudicated claims for more than 190 million unique patients across the US. Adults with ≥1 claim for HMAs between July 1, 2020 and November 30, 2021, and continuous enrollment for 6-months prior to and ≥1-month following the index date were included. Patients newly initiating HMA therapy were identified, where index was the date of the first claim for DEC-C (DEC-C-new cohort) or IV/SC HMA therapy (IV/SC-new cohort), and patients had no claims for HMA therapy in the prior 6 months; a third cohort including all patients initiating DEC-C as either new HMA therapy or switching from IV/SC HMAs was also identified, where index was the date of first claim for DEC-C (DEC-C-all cohort). Patients in the IV/SC-new cohort also had to have ≥1 diagnosis codes for high-grade MDS lesions and/or refractory anemia with excess blasts. Patients had a variable follow-up period of up to 1-year post-index and were followed through December 31, 2021. Demographic/clinical characteristics and treatment patterns were evaluated across the 3 cohorts according to HMA therapy.
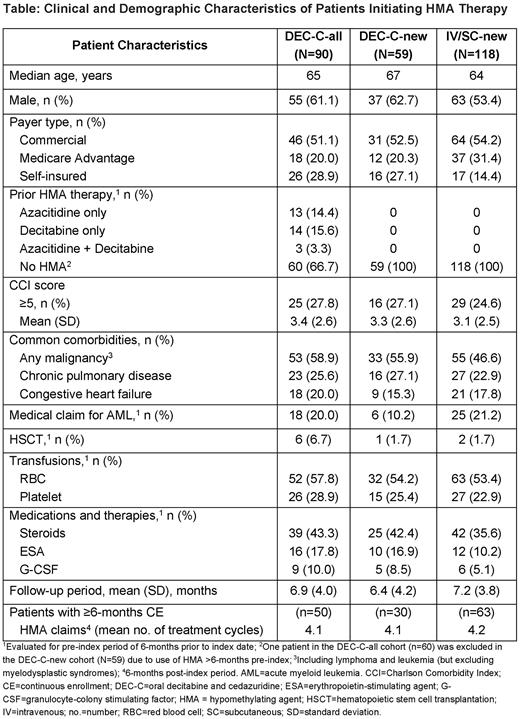
Results: Of 208 patients meeting inclusion criteria, 90 initiated DEC-C (DEC-C-all) and 118 initiated IV/SC HMAs (IV/SC-new) during the study period; 59 patients newly initiated HMA therapy with DEC-C (DEC-C-new). Demographic and clinical characteristics were similar across the 3 cohorts (Table), with no statistically significant differences observed between the DEC-C and IV/SC cohorts newly initiating HMA therapy. Overall, the proportion of males was higher (vs females) across the DEC-C-all, DEC-C-new, and IV/SC-new cohorts (61.1%, 62.7%, and 53.4%, respectively), and most patients were aged ≥55 years (93.4%, 91.5%, and 80.4%; median age 65, 67, and 64 years, respectively). All 3 groups had high levels of comorbidity, with mean Charlson Comorbidity Index (CCI) scores of 3.4, 3.3, and 3.1, respectively, and 27.8%, 27.1%, and 24.6% having CCI scores ≥5. Across the DEC-C-all, DEC-C-new, and IV/SC-new groups, common comorbidities included malignancy (58.9%, 55.9%, and 46.6%, respectively), chronic pulmonary disease (25.6%, 27.1%, and 22.9%) and congestive heart failure (20.0%, 15.3%, and 17.8%). In all groups, some patients had a diagnosis of acute myeloid leukemia (AML) during the pre-index period (20.0%, 10.2%, and 21.2%, respectively), and a small proportion received hematopoietic stem cell transplantation (HSCT) pre-index (6.7%, 1.7%, and 1.7%). Red blood cell transfusions were reported in 57.8%, 54.2%, and 53.4%, and platelet transfusions in 28.9%, 25.4%, and 22.9%, for the DEC-C-all, DEC-C-new, and IV/SC-new groups, respectively. Among patients with ≥6-months continuous enrollment post-index, mean number of HMA cycles of therapy (based on number of claims) over the 6-months post-index were similar (4.1, 4.1, and 4.2 cycles in the DEC-C-all, DEC-C-new, and IV/SC-new cohorts, respectively).
Conclusions: To our knowledge, this study provides the first real-world data on treatment patterns and characteristics among MDS patients initiating oral DEC-C. Characteristics were similar among patients initiating DEC-C and IV/SC HMAs; data also suggest ongoing off-label use of HMAs post-HSCT transplant and in AML. The similar number of HMA claims between groups suggests comparable compliance with oral therapy at home vs IV/SC treatment in the clinical setting, with a possible advantage of DEC-C in reducing the treatment burden associated with existing IV/SC HMA therapy.
Disclosures
Zeidan:Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria. Divino:Taiho Oncology, Inc.: Other: Employee of IQVIA, who received funding from Taiho Oncology, Inc./Epstein Health for this study. DeKoven:Taiho Oncology, Inc.: Other: Employee of IQVIA, who received funding from Taiho Oncology, Inc./Epstein Health for this study. Wang:Taiho Oncology, Inc.: Other: Employee of IQVIA, who received funding from Taiho Oncology, Inc./Epstein Health for this study. Chen:Taiho Oncology, Inc.: Other: Employee of IQVIA, who received funding from Taiho Oncology, Inc./Epstein Health for this study. Salimi:Taiho Oncology, Inc.: Current Employment. Epstein:TriAxia Health: Consultancy; Taiho Oncology, Inc.: Consultancy; Health Rhythms: Consultancy; Tasso inc: Consultancy; Avalon Healthcare: Consultancy; Halozyme: Consultancy; Merck: Consultancy; Janssen: Consultancy; Radius Health: Consultancy; G1 Therapeutics: Consultancy; Illumina: Membership on an entity's Board of Directors or advisory committees; Veracyte: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Membership on an entity's Board of Directors or advisory committees; Diadem: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Decitabine and cedazuridine (ASTX727, DEC-C), is indicated for treatment of adult patients with myelodysplastic syndromes (MDS), including previously treated and untreated, de novo and secondary MDS with the following French-American-British subtypes (refractory anemia, refractory anemia with ringed sideroblasts, refractory anemia with excess blasts, and chronic myelomonocytic leukemia [CMML]) and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups. This abstract reports a study of real-world treatment patterns and may reflect possible off-label use of DEC-C in patients outside of this indication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal